
Our News
RNAi Therapeutics: 5 Things You Should Know about this Innovative Class of Medicines
August 26, 2025
A new era in medicine has arrived. As researchers unravel genes that drive diseases, new treatments called genetic medicines have emerged to target the root causes of disease.
One of the most exciting medical advances is the development of a class of genetic medicines based on a natural process called RNAi interference (RNAi), known as RNAi therapeutics. Here is what you need to know about RNAi therapeutics and how they are transforming the treatment of disease for thousands of patients worldwide.
1. They are Based on Nobel Prize-Winning Discoveries and Inspired by Nature
The naturally occurring process called RNA interference (or RNAi), was first observed in petunias during studies of how genes control flower colors. Researchers found a gene responsible for the petunia’s purple color and thought adding more copies of the gene would result in darker purple flowers. That didn’t happen, and some petunias turned completely white. The reason behind the muting of the purple pigment gene was a mystery for about a decade.
Another group of scientists then conducted similar experiments in a type of worm called C. elegans. They theorized that introducing a specific type of RNA could mute specific genes much like a dimmer switch is used to control lights. Their experiments proved this theory to be correct and earned them the Nobel Prize in Physiology or Medicine in 2006. As exciting as the discovery was, it was uncertain whether RNAi could ever be used to treat disease, but the 1998 paper these scientists published inspired Alnylam’s founders in 1999 to attempt to translate the laboratory discovery of RNAi into medicines for patients. Based on Alnylam's founders work, the company was founded in 2002 to develop this new class of medicines based on RNAi.
Click here to learn more about the discovery of RNAi.
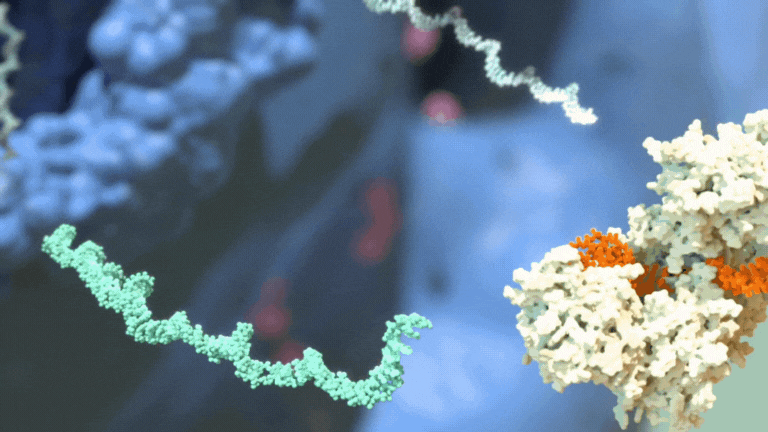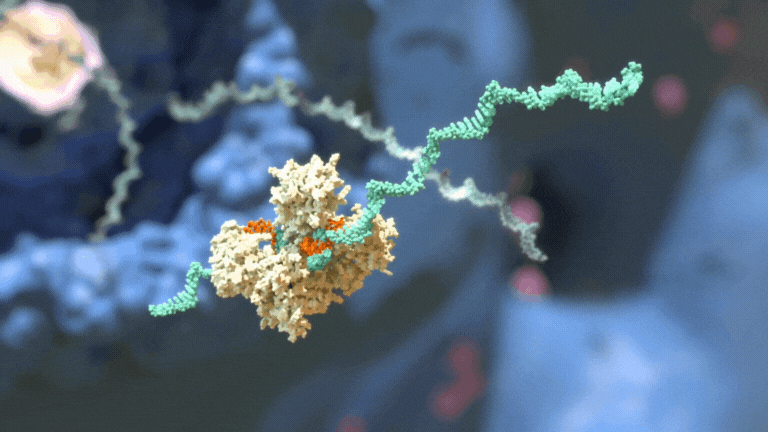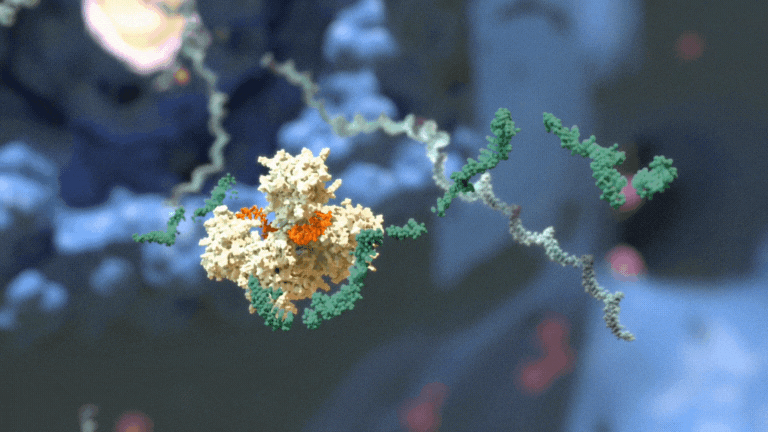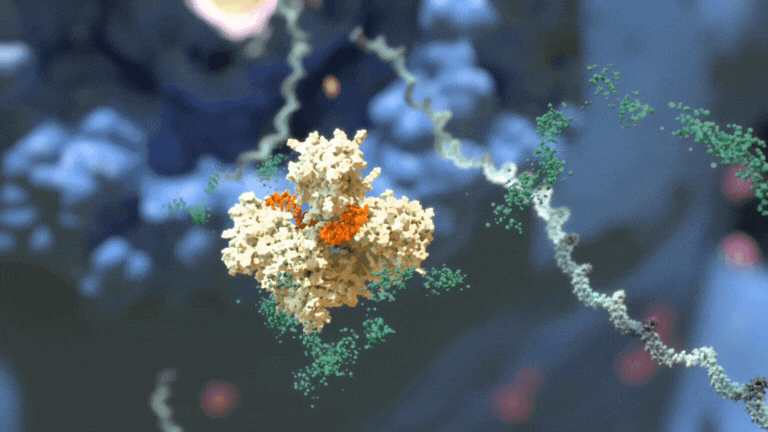 RNAi therapeutics destroy specific mRNA to prevent proteins from being made.
RNAi therapeutics destroy specific mRNA to prevent proteins from being made. 2. RNAi Therapeutics Silence the Genes that Cause Disease
Every cell in our bodies uses RNA interference – think of it like dimmer switch – to control gene expression, adjusting the level of proteins they make. Genes are made of DNA and contain instructions for cells to make proteins. Proteins are the “workers” of cells, such as enzymes, hormones, and more – but proteins can also cause or contribute to disease.
By interfering with the production of unwanted proteins, RNAi therapeutics target disease at its roots. This approach has the potential to silence virtually any gene in the genome. RNAi therapeutics have the ability to slow or halt disease progression by interfering with the production of specific, disease-associated proteins. Thus, these gene silencing medicines are tools for “turning down the volume” of disease-causing proteins inside our cells.
Click here to dive deeper into how RNAi therapeutics work.
3. RNAi Therapeutics Have Advantages Compared to Other Medicines
Most medicines treat the symptoms of diseases rather than the causes. RNA interference therapeutics act before unwanted proteins are made. If disease is compared to a leaking faucet, RNAi is like tightening a faucet to prevent a leak rather than mopping the floor after a leak has occurred.
RNAi therapeutics are also longer-lasting than many other types of medicines, some of which must be taken daily. In contrast, a single dose of an RNAi therapeutic can last for months. Longer-lasting medicines help address the problem of patients missing doses, which has the potential to lead to better health outcomes and a better quality of life for patients.

4. They are an Established Class of Genetic Medicines
Tens of thousands of patients around the world are already being treated with RNA interference therapeutics. There are currently seven FDA-approved RNAi therapeutics, six of which were discovered by Alnylam. While Alnylam has delivered the major breakthroughs and innovations that pioneered this growing class of medicines, other companies are also working to bring additional RNAi therapeutics to patients in need.
Click here to explore Alnylam's timeline of pioneering RNAi advances.
5. RNAi Therapeutics Are Redefining How Diseases Are Treated
We are only at the beginning of what’s possible with RNAi. Alnylam and the other companies now working on RNA interference aim to reach millions of patients living with difficult-to-treat diseases in the coming years. Many clinical trials are underway to explore how RNAi therapeutics may be able to treat a range of cardiovascular diseases, bleeding conditions, and neurological diseases such as Alzheimer’s disease and Huntington’s disease.
Many diseases – rare and prevalent – have limited or no treatment options. The potential for RNAi therapeutics to treat a growing list of diseases represents one of the most exciting and promising advances in medicine.
As the pioneer in RNAi and RNAi therapeutics, Alnylam has, and will continue to, proudly lead the way.
Want to dig deeper into the science of RNA interference? Click here.





