
Our News
First Consensus Recommendations for Holistic Care of People Living With Hereditary ATTR Amyloidosis
September 6, 2023
Before my career brought me into the pharmaceutical industry, I worked as a nurse, first in multiple sclerosis research and then as lead nurse at the UK National Amyloidosis Centre from 2008. I clearly remember my first conversations with patients with hereditary/variant ATTR (hATTR or ATTRv) amyloidosis and their family members and I was truly moved by their stories, their courage and resilience in the face of this relentlessly progressive condition. The impact of the disease on all aspects of life—for the patient and for their caregivers and families—was apparent. I remember being confused and frustrated by the limited treatment options and lack of supportive care.
Fortunately, while the outlook for patients was quite bleak back then, recent years have seen significant advances in research and innovation in treatment development, raising awareness of ATTRv, and action from patient, professional and industry organizations in calling for better management and access to care for those living with the condition. However, despite this, the unmet needs for patients and those caring for them remain high. Treatment is just one element of the equation for ATTRv, as the complexity and severity of the condition means that patients and their families can need many other forms of support. This includes allied health services such as rehabilitation and physiotherapy, nutritional advice, psychological support, and adapted home and workplaces. Without this integrated support in place, people with ATTRv will not experience the improvements in outcomes and quality of life that could be possible.
A large part of managing hATTR amyloidosis is about striking the right balance between trying to ensure a patient feels as comfortable as possible by managing their symptoms, while giving treatment that might slow the amyloidosis down; and that requires holistic, multidisciplinary care. I think you need to find out what’s the most important thing from the patient’s perspective, what’s the most disabling, what’s really impacting on their physical health, their psychological health, and so on, and then address that.” – Dr. Simon Gibbs, amyloidosis specialist and chair of the Australian Amyloidosis Network
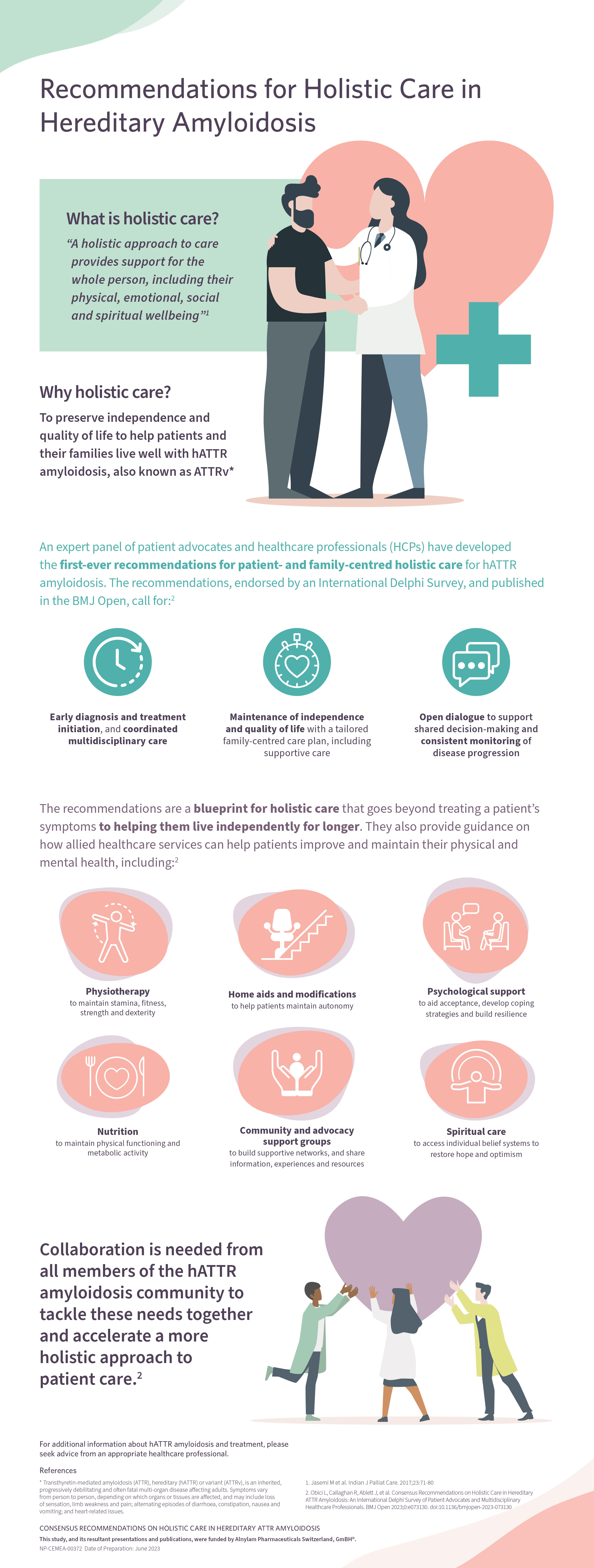
In 2019, the international patient organization Amyloidosis Alliance set out a call-to-action for a broader approach to health and social care to reduce the burden of the disease on patients and their families. I’m delighted to say that this has now been recognised with the publication of the first-ever consensus recommendations for patient- and family-centred holistic care in ATTRv.
The recommendations, published in BMJ Open, were developed by an international expert panel of HCPs and patient advocacy groups and endorsed via a robust international Delphi Survey. They recognize several key areas where improvements in patient care are urgently needed, including:
1. Early diagnosis and treatment initiation, and coordinated multidisciplinary care
2. Maintenance of independence and quality of life with a tailored family-centred care plan, including supportive care
3. Open dialogue between HCPs and patients to support shared decision-making and consistent monitoring of disease progression
It’s important to monitor the patient in all areas, including the holistic care… The patient has symptoms, but they also have a family, a life, and other needs that treatments don’t cover." – Catilena Bibiloni, Asociación Balear de la Enfermedad de Andrade, Palma de Mallorca, Spain; patient and member of the Primary Consensus Panel
I’m incredibly proud to have been involved in this project since its initiation two years ago and as a member of the multidisciplinary primary consensus panel. With the development of these recommendations, the international amyloidosis community have spoken with a unified voice. Now it is time to act and for all groups – patient advocates, HCPs, industry, policy makers - to work together to champion and embed a holistic approach in clinical practice to create a brighter future for patients and those who care for them.
This study, and its resultant presentations and publications, were funded by Alnylam Pharmaceuticals.





