
Our News
Alnylam BioVenture Challenge: Supporting the Next Generation of Biotech Leaders
February 12, 2026
Building a great biotech company is no easy feat. It takes years of research, planning, collaboration, and, of course, investment. Unfortunately, like most start-ups, most biotech’s fail – not for a lack of innovative ideas, but because of the complexity of the industry.
Alnylam has been extremely fortunate. Despite major setbacks early in its 24-year history, our company has persevered and thrived with six Alnylam-discovered medicines approved, a presence in more than 20 countries around the world, and a robust pipeline full of promise.

Supporting the Next Generation of Biotechnology Entrepreneurs
We believe that with our great success comes great responsibility to help others who have ideas that have the potential to transform human health. We recognize that as we continue to grow, we have the opportunity to help up-and-coming biotech entrepreneurs bring their ideas, and companies, to life. That’s why we’ve supported the Nucleate Activator Program since 2022. The program provides young biotech entrepreneurs with workshops, mentorship, and funding opportunities through pitch competitions, like Alnylam’s BioVenture Challenge.
Alnylam’s BioVenture Challenge Pitch Competition
Alnylam and Nucleate worked together to launch the Alnylam BioVenture Challenge in 2022 with the goal of deepening engagement with young biotech entrepreneurs, providing an opportunity for them to learn from Alnylam leadership and earn much needed funding to help them realize their ambitions. Taking place semi-annually, the pitch competition brings together the winners of Nucleate’s Regional Activator Program competitions to compete for a $100,000 grand prize to be used to fund development of their company.
With our most recent BioVenture Challenge held in October of 2025 at our headquarters in Cambridge, MA, we focused on providing a high-value pitch experience for competing entrepreneurs where they could benefit from the scientific and business expertise of Alnylam leaders. We didn’t just want them to present their ideas to us; we wanted them to walk away with actionable feedback and insights.
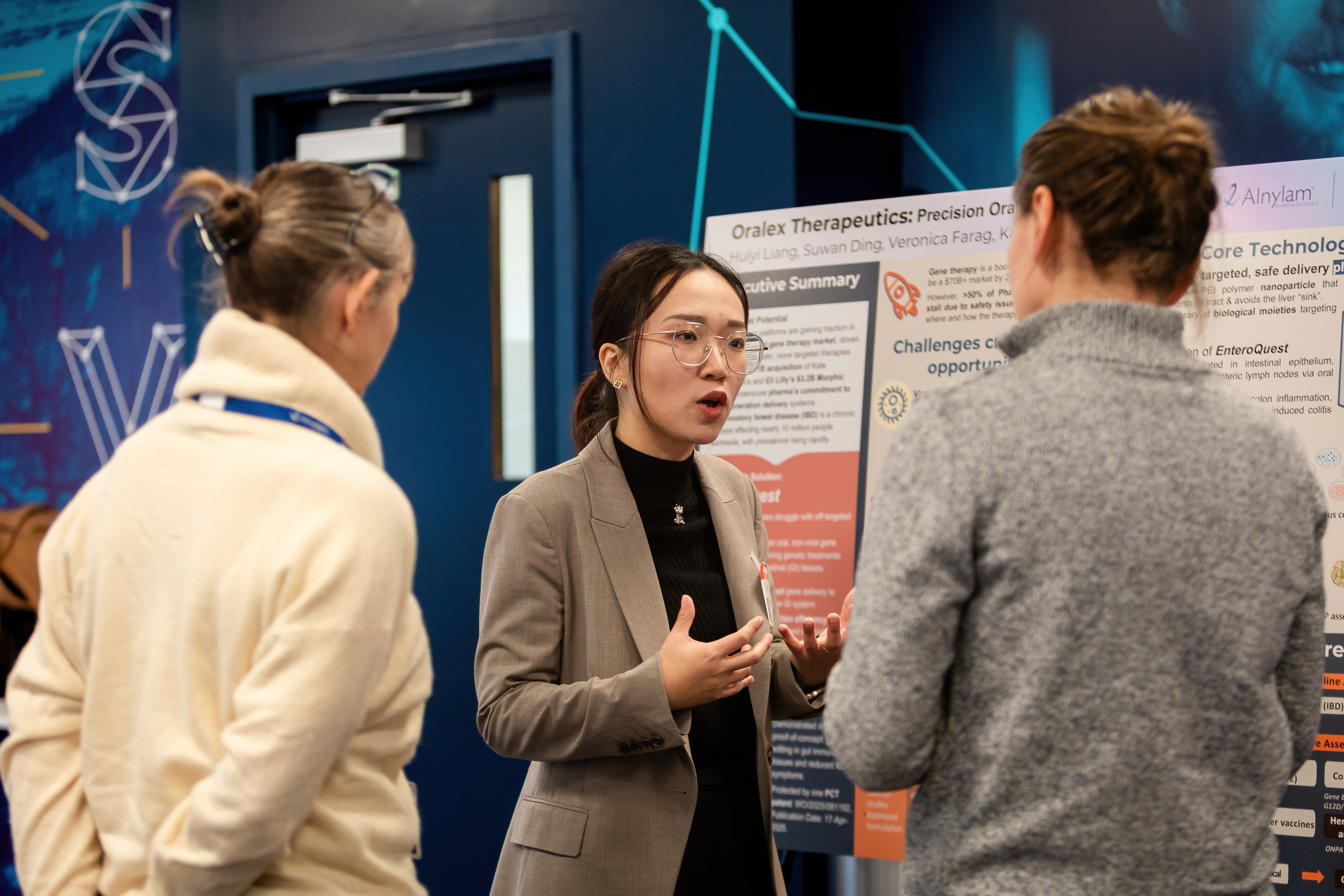 Huiyi Liang, Postdoctoral Fellow, Columbia University, shares about next-generation oral gene delivery platform Oralex.
Huiyi Liang, Postdoctoral Fellow, Columbia University, shares about next-generation oral gene delivery platform Oralex.An Impressive Field of BioVenture Challenge Finalists
We worked closely with Nucleate to identify eight semifinalists selected for their potential to make an impact with their innovative approaches to treating disease. This year’s competing teams included: Elenae Therapeutics, General Control, Neo Splice Therapeutics, Nexendia, Oralex, Pi Bio, Procure Bio, and Sculpta, Inc.
The teams first participated in a poster session, sharing their research with a group of senior Alnylam scientists who helped to narrow the field down to three finalists. Our scientists spent time connecting with each of the teams, asking questions and sharing ideas for them to consider as they pursue their ventures.
From that group of eight, three finalists then pitched their business plans to a panel of Alnylam senior leaders, including our Chief Research & Development Officer Pushkal Garg, Chief Scientific Officer Kevin Fitzgerald, Chief Technical and Quality Officer Tim Maines, and our Head of Business Development Michelle Li.
The competition was intense with presenters fielding questions about their science, business model, expectations for growth, and more. In the end, Sculpta, Inc. earned the top spot along with the $100,000 Alnylam Science and Entrepreneurship grand prize with each of the runners-up receiving a $10,000 prize for their efforts.
 Alnylam’s R&D leaders presenting the $100,000 grand prize to Sculpta, Inc. at our 2025 BioVenture Challenge.
Alnylam’s R&D leaders presenting the $100,000 grand prize to Sculpta, Inc. at our 2025 BioVenture Challenge.
Ours was just one of dozens of similar events that Nucleate hosts each year with corporate partners. It’s their combination of passion, purpose, and great partnerships that enables them to meaningfully scale their impact. We’re proud to partner with this incredible organization and look forward to continuing our work together to amplify ideas that have the potential to shape the future of human health.





