Our News
Harnessing Human Genetics to Power the Next Wave of RNAi Therapeutics
March 10, 2023
Alnylam Pharmaceuticals
Drug development is a perilous process characterized by big risks, large investment (in the order of billions of dollars) and low success rates. The harsh reality is that most investigational drugs (80-90%) do not result in approved medicines. For both drug companies and hopeful patients, this failure rate is daunting. But what if there was a way to improve the likelihood of success? Fortunately, there is… and it has to do with our genes.
One of the tenets of Alnylam’s drug development philosophy from day one, has been to pursue drug targets that have been “genetically validated”, significantly improving their probability of success.
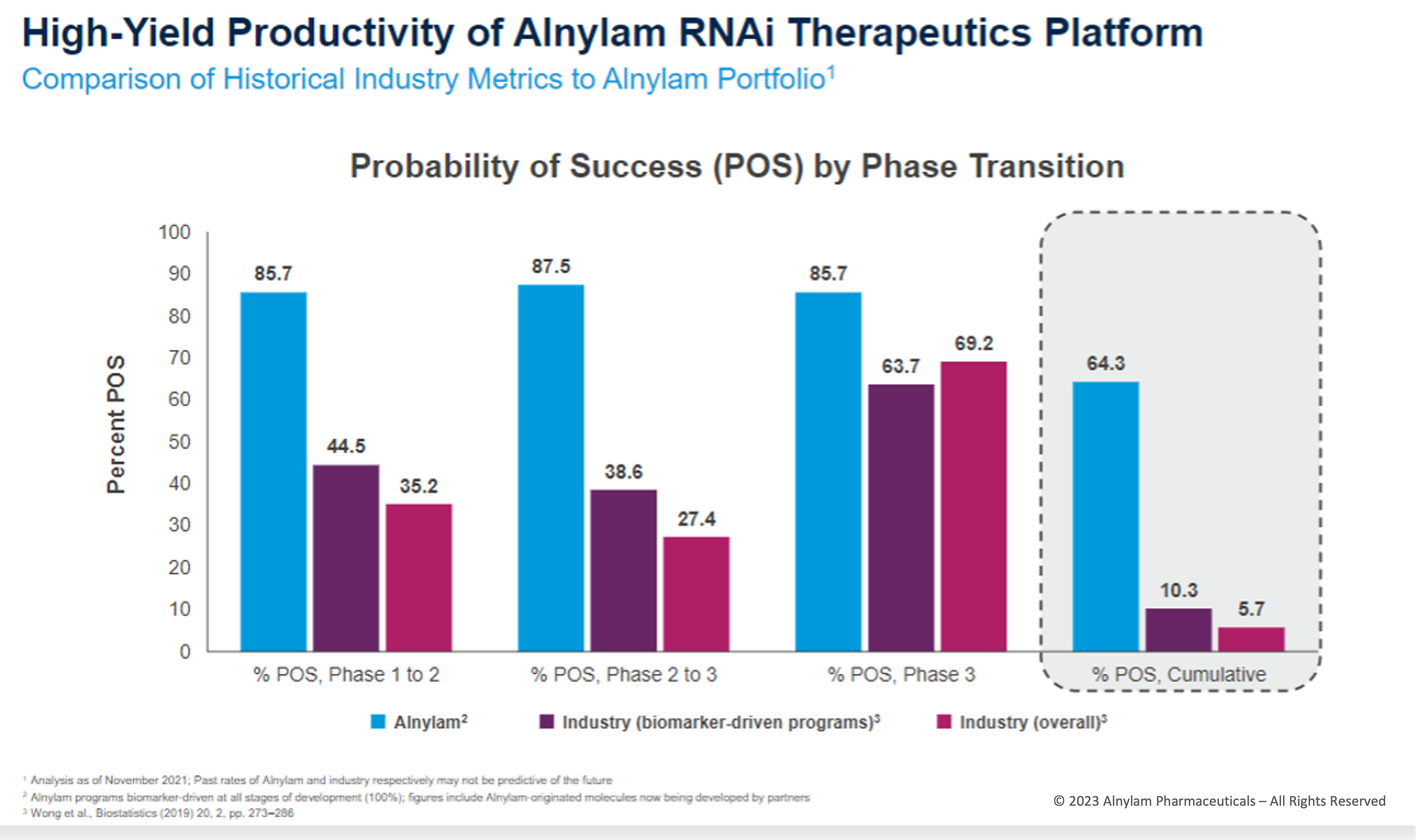 Genetic Validation of Targets Has Yielded Success Rates >6x Industry Avg.
Genetic Validation of Targets Has Yielded Success Rates >6x Industry Avg.
But what do we mean by genetically validated?
When a target is genetically validated it means that there is direct evidence to suggest that changes in that target’s gene sequence are associated with either causing a particular disease or protecting an individual from developing that disease.
The pursuit of drug targets for conditions with a genetic link is more likely to lead to success in clinical trials versus targets which are selected based on knowledge from animal or cell models of disease. For much of the history of drug development, large scale genetics projects were limited to model organisms like rodents or microorganisms. The advent of large-scale genetic testing in humans created the opportunity to understand the direct interaction between gene sequence variants and human health, rather than infer them from disease models in other species. Since RNA interference (RNAi) mimics either the complete or partial loss of function of genes identified in human genetics studies, consequences of silencing those genes are well predicted based on those studies. In short, the traditional approach to target selection for tackling disease involves significantly more guess work and trial and error.
The beauty of using a sequence-based therapeutic approach, like RNAi therapy, is the ability to develop medicines that target any given gene in the human genome that may be implicated in disease. This targeting power coupled with insights on how changes in the sequence of DNA influences the risk of developing a disease over the course of life allows us to continue to pinpoint novel drug targets that we know are core to human disease and develop RNAi-based medicines with a high probability of succeeding in the clinic." – Paul Nioi, PhD, Vice President, Discovery & Translational Research, Alnylam Human Genetics
So how does genetic validation actually work in practice?
The answer begins with the Human Genome Project. Launched in 1990 and completed in 2003, the project has helped scientists decipher the exact sequence of every single gene in your body (your genome), including the specific sequence of all genes that can be involved in driving human disease. The substantial private and public investment in the DNA sequencing technology in turn drove the price of DNA sequencing low enough to enable large scale projects where millions of individuals have had their genomes analyzed.
Using this genome data, scientists can explore the role specific genes play in the origin of diseases they wish to treat. But having this information alone is not sufficient; scientists need a more holistic picture to help them correlate genotype (gene sequence) with phenotype (a patient’s clinical presentation). This is where mining large libraries of genetic information tied to detailed health records of many thousands (and more recently millions) of individuals, comes in handy. Why? Because it turns out that these resources can not only identify genes that may be disease-causing but also variations of genes that are protective for certain health conditions. The precision and sequence-based specificity of RNAi in re-creating these genetics conditions underlies the much greater probability that RNAi therapeutics will have their intended effect in clinical trials.
Within Alnylam, our Alnylam Human Genetics group is charged with leading the genetic validation process and serves as our genetics center of expertise. The group leverages these large sets of genetic data and the latest analytical and computational technologies combined with the expertise of a multi-disciplinary team to inform our research efforts.
Investment in the UK Biobank
The UK Biobank is one of the principal genetics libraries we utilize for the purposes of target identification. Comprised of deidentified health and medical profiles of 500,000 volunteer participants in the UK, the UK Biobank provides researchers a wealth of genomic information at an individual level. In 2018, Alnylam along with several industry peers formed The UK Biobank Exome Sequencing Consortium (UKB-ESC), which is a group of biopharmaceutical companies who endeavored to work with the UK Biobank to provide support and the significant funding required to sequence the whole exomes (the parts of the genome that encode information for protein synthesis) of the 500,000 volunteer participants – a mission that the UK Biobank has now fulfilled as of July, 2022. This initiative offers the unprecedented opportunity to understand how variations in the sequence of DNA influence the risk of developing a disease over the course of life, and to develop medicines that leverage these insights. Under the auspices of the UKB-ESC, this rich data set is now available to researchers around the world upon application at no cost to them.
Example of Insights on Genetically Validated Targets Derived from UK Biobank
Last spring, The Alnylam Human Genetics group and collaborators uncovered that individuals who carry loss of function mutations in a gene called INHBE have reduced abdominal fat, a favorable metabolic profile, and are at lower risk of cardiovascular disease and type 2 diabetes. This compelling discovery utilized sequencing data from more than 360,000 individuals in UK Biobank, and was published in the 13th issue of Nature Communications. Leveraging this insight Alnylam is now pursuing INHBE as a therapeutic target for cardiometabolic disease.
It’s wonderful to see that our partnership with UK Biobank is yielding novel targets in highly prevalent diseases where the unmet need continues to persist. Discovery of INHBE as a potential therapeutic target in cardiometabolic disease is a prime example of what learnings are possible and how much more we are poised to discover by having access to population level genetics.” - Greg Hinkle, PhD, Vice President, Research Informatics at Alnylam
Fueling the Future of Drug Discovery
Genetic validation and the use of genetics databases to fuel drug discovery not only have implications for developing RNAi-based medicines that have a higher probability of success in terms of gaining regulatory approval, but also in terms of having a broader impact on global population health. To that end, in 2022, Alnylam became a Founding Industry Member of Our Future Health – another large-scale (5M individuals) genomic health initiative being led by the UK’s National Health Service (NHS), with Alnylam’s very own, Dr. Paul Nioi appointed as the Chair of the Founders Board in 2023.
Leveraging human genetics to inform the drug development process has always been Alnylam’s approach and we believe, key to our success. As the field of human genetics expands and sophisticated new tools become available to researchers, we are proud to be helping lead the way because at the end of the day, we know that patients are waiting.
To learn more about our genetics approach and hear more in depth from Dr. Paul Nioi, listen to this episode of The Genetics Podcast. We also invite you to learn more about the science of RNAi therapeutics and explore our pipeline.





